What is Medical Device Design Control ?
The objective of medical device design control, is to require that manufacturers follow a methodologically-sound process to develop a medical device, with the intent of improving the probability that the device will reach an acceptable level of efficacy and safety.
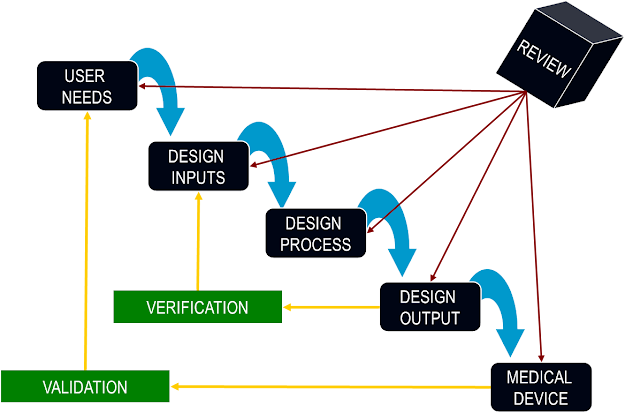
It can be easily understood by considering the waterfall/cascade model in development of medical devices:
The above design cycle is based on the design and development section found in ISO 13485 and ISO 9001 (i.e., – Clause 7.3). It is often mandatory (by regulation) to implement such practice when designing and developing products within regulated industries (e.g. Medical Devices).
The requirement analysis which includes user input documentation and user output documentation are the most crucial steps in designing as they forms the basis of the design.
“ Design is the prolonged checking, pondering, and compromising on requirements which are often quite contradictory until there appears – as the end product of numerous associations of ideas, a network of ideas – the design. ” -Engineering Design, I. R. Matousek
US requirements are outlined in the Quality System Regulation (QSR), which is part of the Code of Federal Regulations. In the context of the QSR and EN ISO 9001, validation ensures fitness for purpose by providing documentary evidence that the device, premises, plant, equipment, process and test methods are capable of functioning and continuing to function to their design and specification.
Validation is concerned with demonstrating the consistency and completeness of a design with respect to the initial ideas of what the system should do. This is often confused with verification, which is concerned with ensuring that, as the design and implementation develop, the output from each phase fulfils the requirements specified in the output of the previous phase.
Since 1990, the Food and Drug Administration (FDA) has required that medical device manufacturers that want to market certain categories of medical in the USA follow Design Control requirements (21 CFR 820.30).
At a high level, this regulation requires:
- Establishment of an intended use and design inputs
- A design plan
- Periodic design reviews throughout the design process
- Confirmation that the design outputs conform to the design inputs through:
- Design verification (“did we design the device right?”)
- Design validation (“did we design the right device?”)
- Translation of the design into manufacturable specifications
- Clear documentation of the entire process in a Design History File or DHF.
References and Further reading:
- Design Control Guidance For Medical Device Manufacturers by fda.
Quality System (QS) Regulation/Medical Device Good Manufacturing Practices by fda